Oppenheimer: The Chemistry Behind Hiroshima and Nagasaki
By: Kaushik Valiveti
On July 21st, 2023, Christopher Nolan’s Oppenheimer both debuted and quickly rose to the top of critics’ lists, stunning the audience with its heart-pounding action sequences, immersive audio design, exceptional performances, and meticulous direction. But to those who haven’t secured a seat yet (which is likely, considering every theatre is consistently booked!), let me give you a run-down of what goes on (spoiler-free, I promise). J. Robert Oppenheimer was one of the greatest minds of science during World War 2 and was selected to spearhead the Manhattan Project (a secret project to test nuclear weapons in the desert of Los Alamos) because of his outstanding brilliance. He fathered the atomic bomb, a creation that cost around 120,000 Japanese lives. However, the successful deployment of the atomic bombs on Hiroshima and Nagasaki marked a turning point in modern warfare and the dawn of the atomic age. But how did Oppenheimer postulate such a destructive bomb all the way in the mid-1900s when the only form of computing was a typewriter? The answer lies in quantum theory and the fundamentals of chemistry brewing in London.
*Watch this video to understand the immense scale of the atomic bomb
A photo of J. Robert Oppenheimer performing mathematic calculations
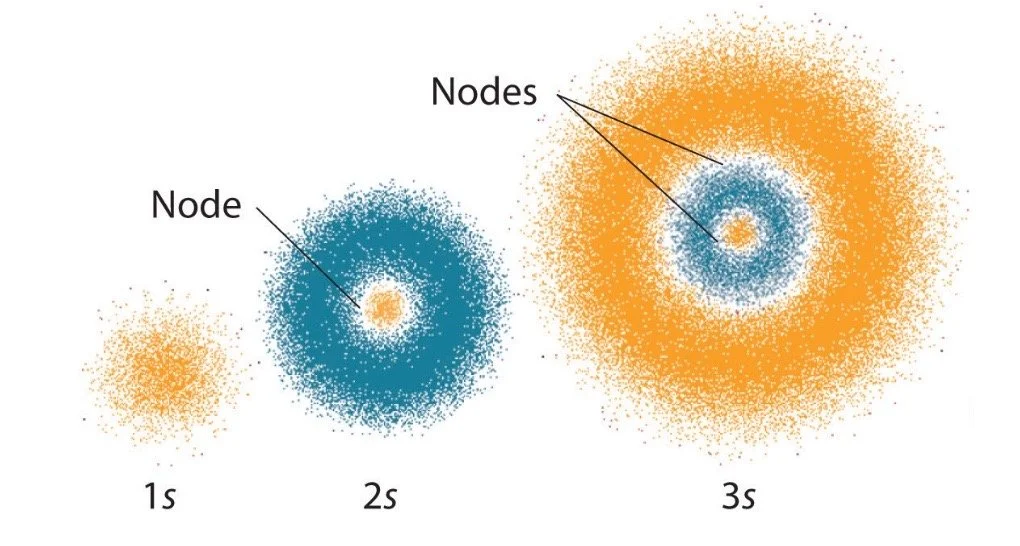
Understanding isotopes and the atomic model is crucial for understanding what went on inside the atomic bomb.
Above is the Quantum Mechanical Model of an atom, created by Werner Heisenberg. He proposed that the atom is not a fixed entity—it is constantly moving and rapidly hybridizing. There are certain “nodes” in the atom where there is the highest probability of finding an electron. The key point to take away is that the atom is unpredictable. Its components are rapidly moving, and we are constantly uncertain where those components lie at a certain time. This point became known as the Heisenberg Uncertainty Principle.
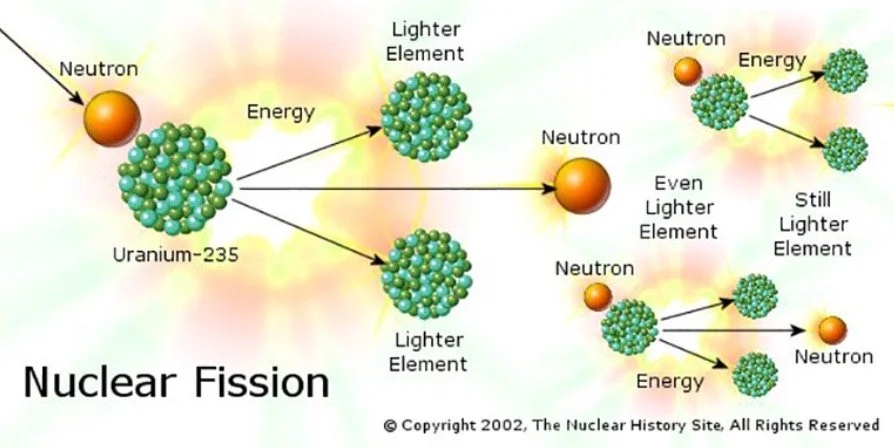
Most of you are aware that the protons and neutrons are at the core of an atom, and the electrons are orbiting this positively charged center. But what if there are more neutrons than protons? This is what’s called an isotope, and because of the imbalance between the two subatomic particles, the atom becomes unstable, impatient at how abnormal the coulombic attractions are between the protons and neutrons. In the atomic bomb, enriched uranium-235 and plutonium-239 served as the fissile material because both uranium and plutonium’s isotopes are highly radioactive (unstable) and can undergo fission very easily in an attempt to stabilize the molecule.
Above is a simplified visual to explain nuclear fission
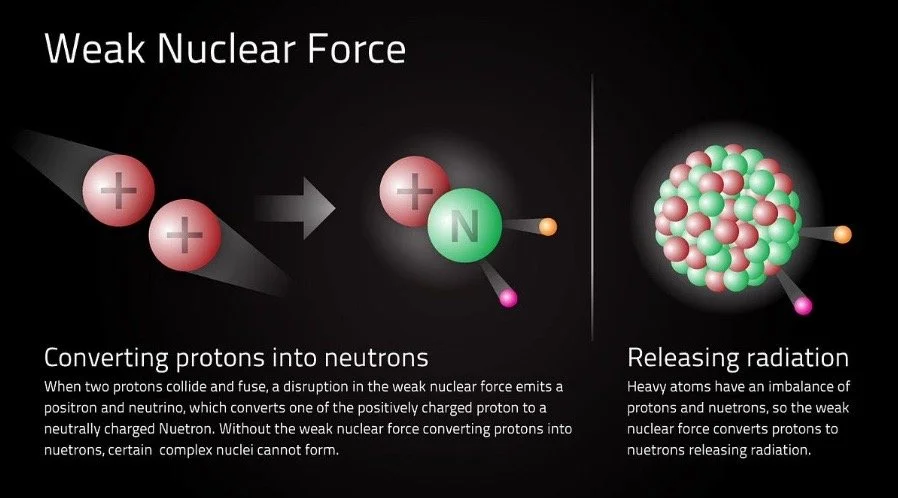
The weak nuclear force is very weak (obviously) but the significance of this is that radioactive isotopes can decay, or emit an electron through alpha or beta decay. If the weak nuclear force was strong and radioactive isotopes were unstable, they could never achieve a state of stability.
As described by the first image, a neutron is ejected at the isotope, and the chain reaction caused by fission unleashes an enormous amount of energy in the form of an explosive blast, a blinding flash of light, and a searing release of radiation. This catastrophic event is the essence of an atomic explosion, and the magnitude of the energy is capable of unspeakable things. And to think that it all starts with one tiny neutron, 100,000 times smaller than the tip of a hair.
Another important aspect of Oppenheimer's atomic bomb was the lengthy time of radioactivity even after the initial explosion. Using modern mechanics, scientists were able to come up with a phenomenon known as nuclear isomerism. In this process, certain isotopes produced during the fission reaction are trapped in a higher-energy state rather than immediately emitting subatomic particles in order to reach the ground state, or the lowest, most stable energy level. These nuclear isomers can release additional energy in the form of gamma radiation hours or even days after the initial detonation.
The chemistry behind Oppenheimer's atomic bomb continues to be studied and refined even today. The chemistry knowledge then used for creating the atomic bomb is now being used for MRI development, chemotherapy, and space exploration. As we navigate the complexities of nuclear technology, history serves as a stark reminder of the potential consequences of wielding such immense power and even how the purest intentions sometimes lead to the most disastrous outcomes.
Works Cited:
“Science behind the Atom Bomb.” Nuclear Museum, https://ahf.nuclearmuseum.org/ahf/history/science-behind-atom-bomb/#:~:text=Fission,a%20tremendous%20amount%20of%20energy.
“Fission and Fusion: What Is the Difference?” Energy.Gov, April 1, 2021, https://www.energy.gov/ne/articles/fission-and-fusion-what-difference#:~:text=Fusion%20occurs%20when%20two%20atoms,produce%20highly%20radioactive%20fission%20products.
“MIT Nuclear Reactor Laboratory.” The Fission Process | MIT Nuclear Reactor Laboratory, https://nrl.mit.edu/reactor/fission-process.