What is String Theory?
Written By: Arman Momeni
Introduction
In 1803, John Dalton revolutionized the world with his creation of atomic theory. Dalton founded the approach that all matter, everything in the world, is made up of these small indivisible particles called atoms. While mostly correct, mankind has learned so much about the atomic world and has developed new theories in understanding just how our world works. Now, for the past 50 years, scientists have been working on a fundamental theory in hopes of defining EVERYTHING, and they call it string theory. String theory proposes that all matter is composed of minute vibrating strings. I know what you’re thinking, it sounds wild, and I agree, but before we get to that, let’s learn a little about the background behind string theory.
The History of Atomic Theory
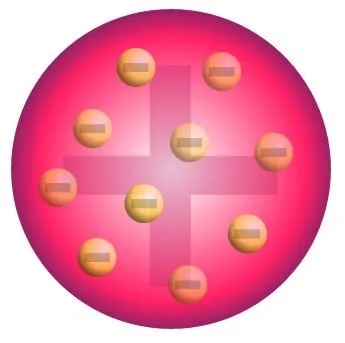
As stated above, atomic theory started with Dalton. His main revolutionary point was that matter is composed of tiny indivisible particles called atoms; however, Dalton only touched the surface. He failed to dive further and explore what resides inside of the atom. Later on, J. J. Thomson, another brilliant mind, proposed that mass and charge were distributed evenly throughout an atom, and he proposed the plum pudding model, founding the electron. In his model, the electrons were the plums, and the pudding was positively charged. The electron was the first sub-atomic (smaller than atom) particle to be discovered. At the time of its discovery, the only information about it was that it held a negative charge, which canceled out the positive charge that comprised the rest of the atom.
J. J. Thomson’s plum pudding model of the atom
The next great discovery was made by Ernest Rutherford. Rutherford set out to prove Thomson’s model, but instead he falsified it through his gold foil experiment. The gold foil experiment consisted of a gold foil, and alpha particles, which were a type of radioactive particle with four times the mass of a hydrogen atom. If Thomson’s model held true, and atoms held a uniform charge, then scientists expected that all of the alpha particles would pass right through the gold foil. To their surprise, a small number of the alpha particles bounced off the foil with heavy obtuse angles. In order to explain his results, Rutherford developed a new model of the atom. Rutherford concluded that atoms were mostly empty space, but they all had a very concentrated small interior, which he called the nucleus. The nucleus, in Rutherford’s theory, is a tiny, dense, and central core of the atom that is composed of protons (positive charge) and neutrons (neutral charge). This model was called the nuclear model.
Rutherford’s Gold Foil Experiment
While Rutherford’s theory mostly held true, there were a couple of flaws. If the protons are all positively charged (and like charges repel each other), why didn’t the nucleus tear apart? Or, if opposite charges attract, why didn’t the negatively charged electrons collapse into the positively charged nucleus?
Niels Bohr provided the answer to the second question, stating that electrons have a fixed energy and orbit around the nucleus. While Bohr’s model explained a lot at the time, we know that his model was not entirely correct.
The Bohr model of the atom
Later on, in 1926 Erwin Schrodinger, an Austrian physicist, took the Bohr model, and advanced it even further. Schrodinger used mathematics to express the probability of finding an electron in a certain position. This model was called the quantum mechanical model of the atom.
The Composition of the Atom:
Now if you are confused by atomic theory, don’t fret. The basics that you need to know is that all matter, as stated above, is composed of atoms. While incomprehensibly small, atoms are not the smallest components of matter. Our current understanding states that atoms are made of protons (positively charged particles), neutrons (no charge), and electrons (negatively charged particles); these are all sub-atomic particles. While neutrons and protons are the same mass, the electron is far smaller. The electron’s mass is so small that it is denoted as negligible (essentially 0). Past the proton, neutron, and electron, are quarks. Quarks are elementary particles that combine to form composite particles called hadrons; protons and neutrons are the most stable type of hadrons. The electron, however, is only composed of a single quark. As of now, quarks are the smallest proven particles, however, string theory attempts to break it down even further.
All of the currently known elementary particles. There are 6 types of quarks!
Quantum Theory + Quantum Physics
Now that we know about the atom, let’s learn about quantum theory. Quantum theory was a result of physicists becoming confused about the behaviour of atoms. After observing the behaviour of atoms in a series of experiments in the late 1800s/early 1900s, physicists concluded that atoms did not make intuitive sense when compared to what they knew about physics.
Quantum theory gets complex, and for the sake of this article, we don’t need to know all the details. Quantum theory, in simple terms, is the theory of matter. Scientists want to know how the small components of matter – atoms and sub-atomic particles – behave, and how that impacts familiar matter, the world of objects that we see.
General Theory of Relativity:
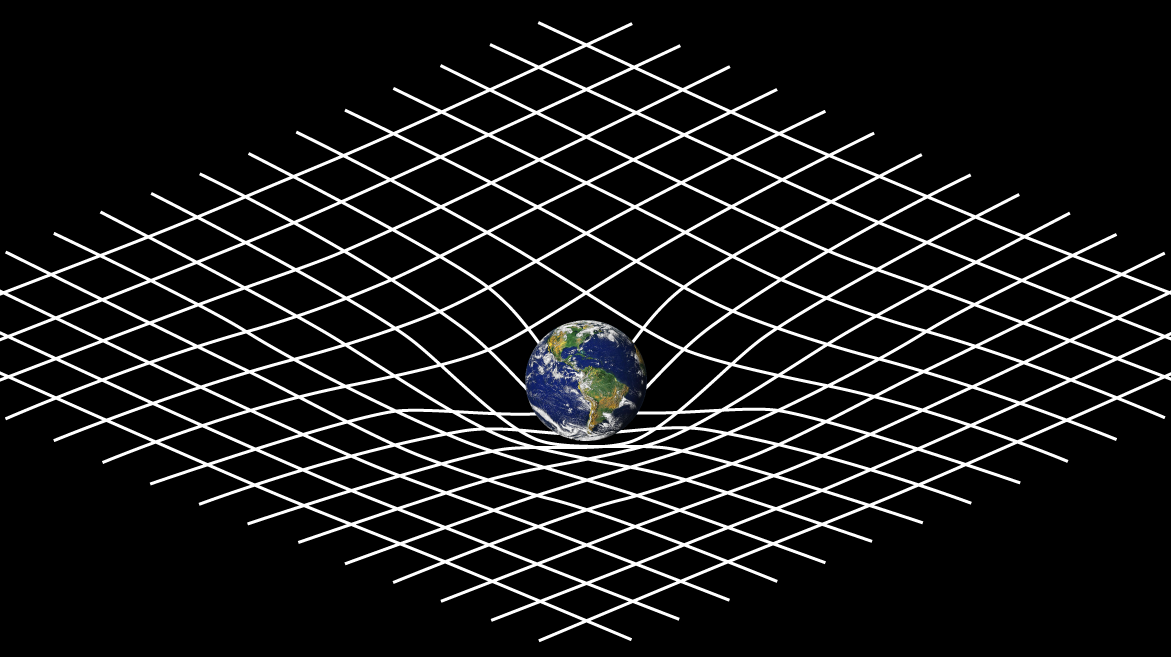
We’ve spent a lot of time talking about the tiny world. Quantum and atomic theory discuss a lot about small things, but they neglect the larger world. General relativity, which was created by Albert Einstein, talks about large things, and discuss how gravity affects space-time (the concept of time and three-dimensional space combined together). Einstein discovered that massive objects warp the fabric of space-time, and this distortion is known as gravity. The theory of general relativity, published in the early 20th century, was quite revolutionary. Einstein’s field equations helped describe situations such as planetary dynamics, the birth and death of stars, black holes, and even the evolution/creation of the universe.
A visual example of the curvature of space-time with respect to general relativity
Putting All Together:
Now we know about two separate theories: general relativity and quantum theory, but how do they connect? The answer is: they don’t, at least not yet. Quantum mechanics has been successful in describing the behaviour of small things, and general relativity has been successful in explaining the behaviour of really large things, but they don’t combine well together. Quantum mechanics contradicts general relativity, and vice versa. String theory is an attempt by scientists to unify these two theories, and help solve some of the conflicts between them. String theory, as stated above, is an idea, which states that matter as we know is not actually made of particles, but instead, is made up of tiny little strings. String theory states that matter and its composition are actually controlled by the varying frequency of these strings.
String theory is more complicated than just strings, however. String theory is a theory of quantum gravity. Scientists want to be able to understand how gravity, and large forces, impact sub-atomic particles. Physicists hope that string theory acts as an outlet to help solve other problems in the world of science.
Nonetheless, despite being worked on for half a century, string theory has yet to be proven. The main trouble with string theory is that the mathematics and concepts only seem to work in a world that has 10 space-time dimensions, whereas our known world only has 4 (length, width, height, and time). Nonetheless, it is not a matter of discovering 6 new dimensions, many scientists believe that these extra dimensions are crammed in a small compact shape, that can only be seen by certain experiments and not the naked eye.
Works Cited:
Development of the atomic theory. (n.d.). http://www.abcte.org/files/previews/chemistry/s1_p6.html#:~:text=In%201926%20Erwin%20Schr%C3%B6dinger%2C%20an,mechanical%20model%20of%20the%20atom.
Britannica, T. Editors of Encyclopaedia (2023, June 28). general relativity. Encyclopedia Britannica. https://www.britannica.com/science/general-relativity
Foundation, C.-12. (n.d.). Gold Foil Experiment . CK. https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/4.14/primary/lesson/rutherfords-atomic-model-chem/
Particles. Simple science: Particles. (n.d.). https://www.imperial.ac.uk/humanities/webdesign/2012/nickyguttridge/html/page4.html#:~:text=A%20quark%20is%20an%20elementary,which%20are%20protons%20and%20neutrons).
Quantum Mechanics history. Maths History. (n.d.). https://mathshistory.st-andrews.ac.uk/HistTopics/The_Quantum_age_begins/
Tillman, N. T., Bartels, M., & Dutfield, S. (2022, January 5). What is the theory of general relativity?. Space.com. https://www.space.com/17661-theory-general-relativity.html
Wood, C., & Stein, V. (2022, January 20). What is string theory?. Space.com. https://www.space.com/17594-string-theory.html